Niels Bohr in 1913 will use the hydrogen spectrum to start on the road to explaining how electrons are arranged in an atom. The colors cannot be expected to be accurate because of differences in display devices.

Emission Spectrum Of Hydrogen Video Khan Academy
So why do energized hydrogen gas molecules produce a line spectrum but not a continuous spectrum.

. The hydrogen atoms of the molecule dissociate as soon as an electric discharge is passed through a gaseous hydrogen molecule. Explaining hydrogens emission spectrum. The energy shells are usually given a letter n to describe the specific energy level.
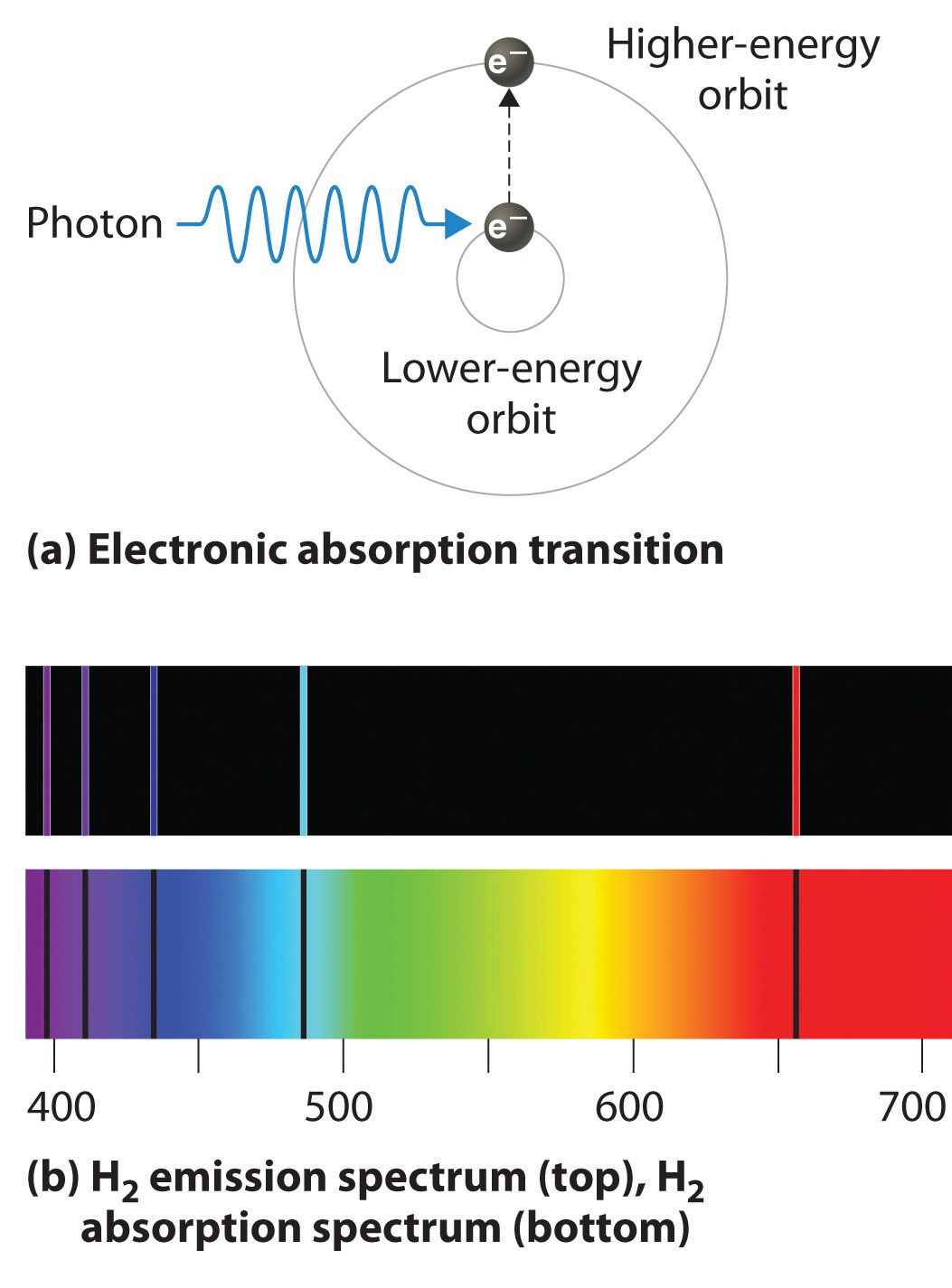
Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels. While the electron of the atom remains in the ground state its energy is unchanged. The left edge ends at a wavelength of 4000 Ångströms and the right edge ends at 7000 Ångströms.
The emission spectrum of hydrogen occupies a very important place in the history of chemistry and physics. When this light is passed through a prism as shown in the figure below four narrow bands of bright light are observed against a black background. The hydrogen emission spectrum consists of radiations of discrete frequencies.
Have students make hypothesis as to why each spectrum is different. When a photon is absorbed by a hydrogen atom the energy of the photon causes the electron to undergo a transition to a higher energy level n 1 n 2 for example. Because of this a line emission spectrum is like the fingerprint of the element which we can use to identify the element.
By comparing the combined spectra to the known spectra. Light Calculations Worksheet example included at end of this lesson. A blue line 434 nanometers.
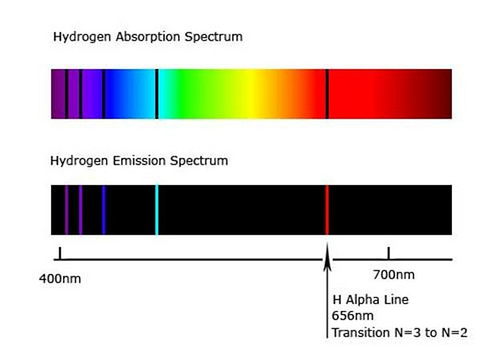
So you see one red line and it turns out that that red line has a wavelength-- that red light has a wavelength of 656 nanometers. Youll also see a blue-green line and so this has a wavelength of 486 nanometers. Atomic theory - The hydrogen emission spectrum and how it provides evidence for the existence of energy levels around the atomic nucleus.
Put a large voltage across the gas. Walk students through the hydrogen emission spectrum and Bohrs model of an atom rather than have them do the calculations. It was realized that a heated gas emits a unique combination of colors called emission spectrum depending on its composition.
This spectrum was produced by exciting a glass tube of hydrogen gas with about 5000 volts from a transformer. According to the Bohr model for the hydrogen atom the energy of the atom is not continuous but has certain discrete energy _____ each of which is related to a fixed circular _____ of the electron around the nucleus. By an amazing bit of mathematical insight in 1885 Balmer came up with a simple formula for predicting the wavelength of any of the lines in what we now know as the Balmer series.
Each element produces its own unique line spectrum. Balmers equation was then expanded to describe the entire spectrum of hydrogen including the ultra-violet and. Further splitting of hydrogen energy levels.
And a violet line at 410 nanometers. The lines that are produced due to the emission from equal to 2 appear in the emission spectrum. Bohrs model explains the spectral lines of the hydrogen atomic emission spectrum.
Bohrs model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells or orbits around the nucleus. This video explains the spectral lines in absorption and emission spectra as well as the hydrogen emission spectrumHelp support this channelhttpswwwpa. It was viewed through a diffraction grating with 600 linesmm.
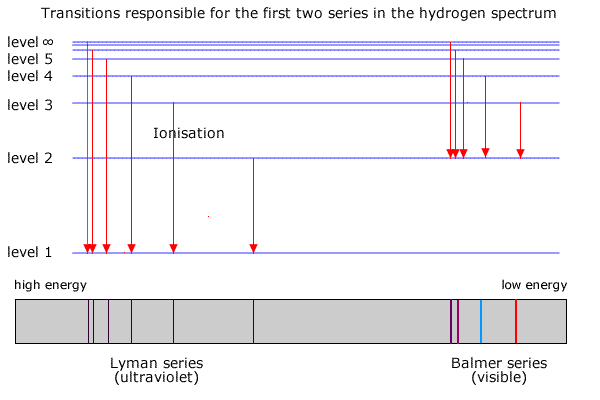
These series of radiations are named after the scientists who discovered them. E that is needed to remove the electron from the first Bohr orbit is called the ionization limit of the hydrogen atom. There are many possible electron transitions for.
Line emission spectrum. The hydrogen spectrum is an important piece of evidence to show the quantized electronic structure of an atom. Since hydrogen is present in the first shell it will always absorb some amount of energy to reach the higher levels.
2 2 Hydrogen Emission Spectrum Sl Youtube Remember me on this computer. To relate the energy shells and wavenumber of lines of the spectrum Balmer gave a formula in 1855. The Lyman Series is a group of lines in the ultraviolet portion of the emission spectrum of the hydrogen atom named after their discoverer Theodore Lyman who found these lines between 1906 and 1914.
The emission spectra of an element is the spectrum of radiation emitted due to an atom or molecule absorbing energies and transitioning from a high energy state to a lower energy state. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. When the radiations emitted by different substances which are produced in.
The emission spectrum of hydrogen shows 4 lines. Three years later Rydberg generalised this so that it was possible to work out the. Pencils to draw the emission spectrum of each gas.
Figure 617 The energy spectrum of the hydrogen atom. Emission Spectrum of Hydrogen. The Balmer and Rydberg Equations.
Atoms molecules or ions that absorb radiation are known to be in an excited state. This series is known as Balmer series of the hydrogen emission spectrum series. Balmer series is also the only series.
So this is the line spectrum for hydrogen. The ionization limit in Equation 647 that we obtain in Bohrs model agrees with experimental value. It will break down and emit light.
Using Balmer-Rydberg equation to solve for photon energy for n3 to 2 transition. In 1961 from University of Tokyo. Helium gas in a discharge lamp.
The photon energy of the emitted photon is equal to the energy difference between the two states. IB Chemistry - Atomic Theory. Bohr had an answer.
Herschels discovery of emission spectra from heated gas was studied extensively in the 1800s. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a transition from a high energy state to a lower energy state. These lines occur when the excited electron falls back from a higher energy level n 2 to the first energy level n1.
If one has a collection of several elements all emitting light spectra of the different elements combine or overlap. It results in the emission of electromagnetic radiation initiated by the energetically excited hydrogen atoms. The Hydrogen Spectrum.
Bohrs model calculated the following energies for an electron in the shell. V 109677 1 2 2 1 n 2 Where v is the wavenumber n is the energy shell and 109677 is known as rydbergs constant. When the atom absorbs one or more quanta of energy the electron moves from the ground state orbit to an excited state orbit that is further away.
One line is in the higher range that is and the other three lines in the range of. Solving for wavelength of a line in UV region of hydrogen emission spectrum.
How Did Bohr Theory Explain The Emission Spectrum Of Hydrogen Socratic

2 2 Hydrogen Emission Spectrum Sl Youtube
Atomic Theory 1 33 The Hydrogen Spectrum
Absorption Spectrum Emission Spectrum Lines Article Khan Academy
0 Comments